Abstract
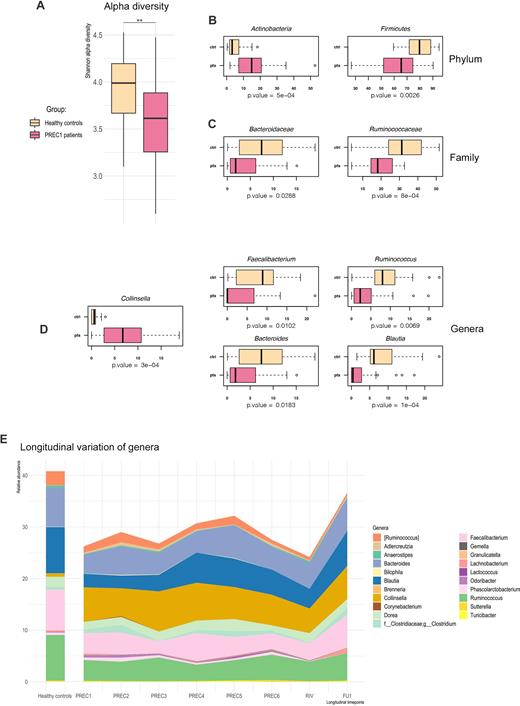
Although front-line R-CHOP (rituximab-cyclophosphamide, doxorubicin, vincristine, prednisone) can improve clinical outcomes in diffuse large B-cell lymphoma (DLBCL), 20%-25% of patients relapse after initial response, within two years. Recent research on the gut microbiome (GM) in cancer has highlighted its importance in hematology. Indeed, GM can impact the onset and progression of cancer, as well as the outcomes of anticancer therapies and drug side effects.Fifty DLBCL patients undergoing front-line R-CHOP therapy were enrolled (ClinicalTrials.gov Identifier: NCT03797170, RF-2016-02363730). Feces were collected at baseline, before each therapy cycle and at response assessment (during both therapeutic course and follow-up). GM was profiled by 16S rRNA amplicon sequencing. Patients responding to first line will be followed up for two years after the end of treatment by fecal sampling every 6 months, in correspondence with disease re-evaluation. The GM of DLBCL patients before starting R-CHOP was compared with already-published data from healthy controls matched by geographical origin (Italy), gender and age. Patients showed less alpha diversity and some changes in composition, including notably an increase in Collinsella and a decrease in typically health-associated taxa, such as Faecalibacterium, Ruminococcus, Blautia and Bacteroides (Figure 1A-D). Concerning the GM dynamics though the R-CHOP therapy cycles (Figure 1E), no significant changes were observed but an overall reduction of several taxa starting from the fourth cycle. The compositional differences reported at baseline persisted until response assessment (following the last therapy cycle), while they partially reversed at the first follow-up (6 months after the end of therapy), with the recovery of healthy-like proportions for Bacteroides, Blautia, Faecalibacterium, Ruminococcus and Collinsella. All but two patients responded positively to therapy.The GM of DLBCL patients showed a significant alteration even before starting R-CHOP therapy, with reduced richness and dysbiotic compositional features. This imbalance was maintained until the fourth cycle, after which we detected an overall GM shrinkage. Six months after the end of therapy, the dysbiotic signatures were largely reversed, suggesting that GM resilience may be involved in the response to therapy.
Disclosures
Zinzani:Gilead: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celltrion: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Secura Bio: Membership on an entity's Board of Directors or advisory committees; Beigene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Incyte: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Kyowa Kirin: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; EUSA Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; MSD: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sandoz: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen-Cilag: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Servier: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal